The first and the only electric-field navigated transcranial magnetic stimulation (TMS) device cleared by FDA for presurgical brain function mapping.
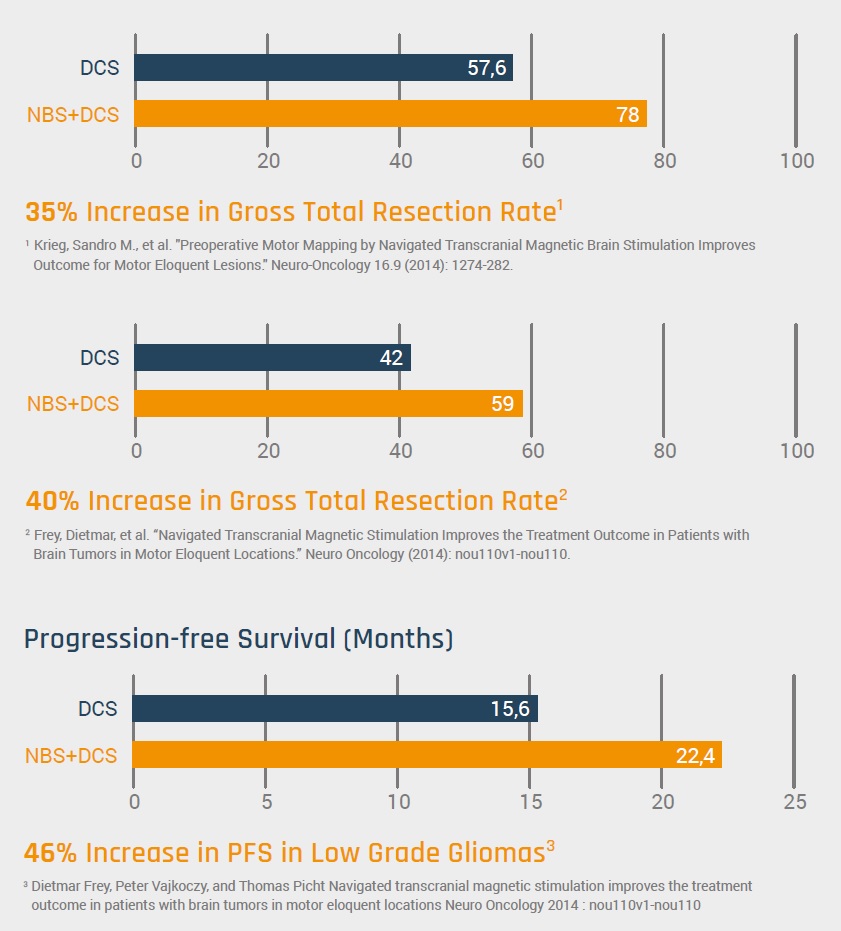
The Nexstim Navigated Brain Stimulation (NBS) system for presurgical brain functions mapping helps neurosurgeons preserve what’s essential. NBS offers neurosurgeons the chance to improve outcomes and patients’ quality of life. The clinical value of using the NBS system has been firmly established, with over 50 papers on over 2300 patients published by 2018. Multiple clinical studies have proven that NBS, as an adjunct to direct cortical stimulation, results in a 35% increase or greater, in the rate of gross total resection [1, 2]. In a recent study of 250 consecutive patients, presurgical mapping with NBS enabled a more aggressive surgical strategy in more the 75% of the cases. The same study using navigated TMS (nTMS) disproved suspected involvement of primary motor cortex in over 25% of the cases, expanding surgical indication by 14.8%. These significant findings conclude that the integration of nTMS into the surgical workflow crucially improves pre-operative planning, patient counseling, and surgical procedures, leading to longer progression-free survival rates [3] and better neurological outcomes by expanding the indications and extent of resection.
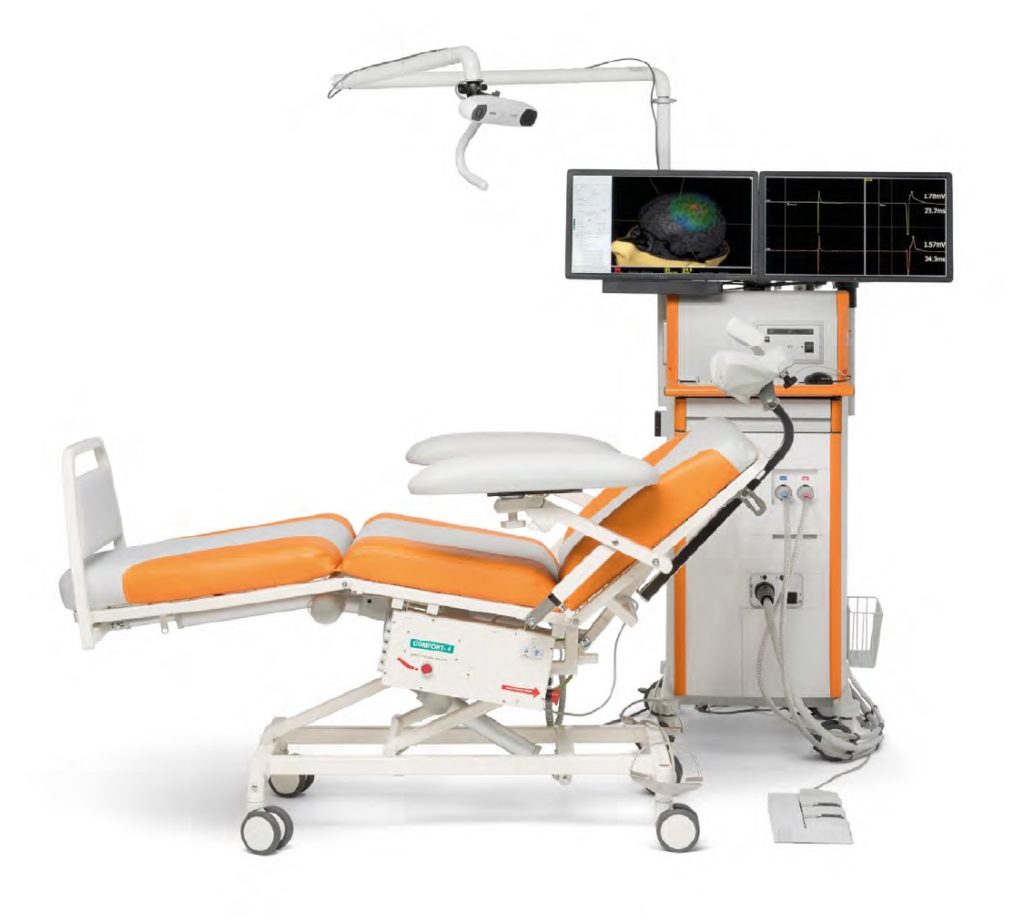
The NBS 5 Motor Mapping System comprises the following components:
- Mobile Nexstim cart
- Nexstim TMS stimulator
- Nexstim stimulation coil
- Stereotactic camera (arm-mounted tracking unit)
- 6-channel Nexstim EMG
- NBS computer system with dual 23” displays, PC and NBS software
- Three-pedal foot switch
- Nexstim cooling unit (optional)
- Electronically-adjustable patient chair
System also includes tracking tools: NBS head tracker, coil tracker and digitizing pen.
NBS 5 Speech Mapping System with NexSpeech®
The NexSpeech® 2 module comprises the following components:
- NexSpeech Presentation Set, including: LCD unit for displaying image stacks to the patient and video camera for recording speech behavior in baseline and stimulation sessions
- NexSpeech Camera and Display Holder
- Single-use NexSpeech Head Tracker
- NexSpeech 2 main software and Analyzer software (installed on NBS System PC)
Nexstim Navigated Brain Stimulation (NBS) System 5 and NexSpeech are cleared by the Food and Drug Administration (FDA, K112881). The Nexstim Navigated Brain Stimulation (NBS) System 5 is indicated for non-invasive mapping of the primary motor cortex of the brain to its cortical gyrus. The Nexstim NBS System 5 provides information that may be used in the assessment of the primary motor cortex for pre-procedural planning. Nexstim NexSpeech®, when used together with the NBS System 5, is indicated for non-invasive localization of cortical areas that do not contain essential speech function. NexSpeech® provides information that may be used in pre-surgical planning in patients undergoing brain surgery. Intra-operatively, the localization information provided by NexSpeech® is intended to be verified by direct cortical stimulation.The Nexstim NBS System 5 and NBS System 5 with NexSpeech® are not intended to be used during a surgical procedure. The Nexstim NBS System 5 and NBS System 5 with NexSpeech® are intended to be used by trained clinical professionals.
